Transcriptomics of Hemocyte Subpopulations in Cherax quadricarinatus
-
摘要:目的
半颗粒细胞(semigranular cell, SGC)和颗粒细胞(granular cell, GC)是螯虾循环血细胞的两大主要类群,它们代表了处于不同发育阶段的具有免疫功能的血细胞。了解SGC和GC的功能,可为深入研究甲壳动物血细胞亚群提供基础数据。
方法以红螯螯虾(Cherax quadricarinatus)为试验对象,运用Percoll不连续密度梯度离心法分离纯化SGC和GC;然后对SGC和GC进行转录组测序,并进行差异分析、GO富集分析以及KEGG富集分析,以探究他们在功能上的差异;最后用实时定量RT-PCR对部分差异基因的表达进行验证。
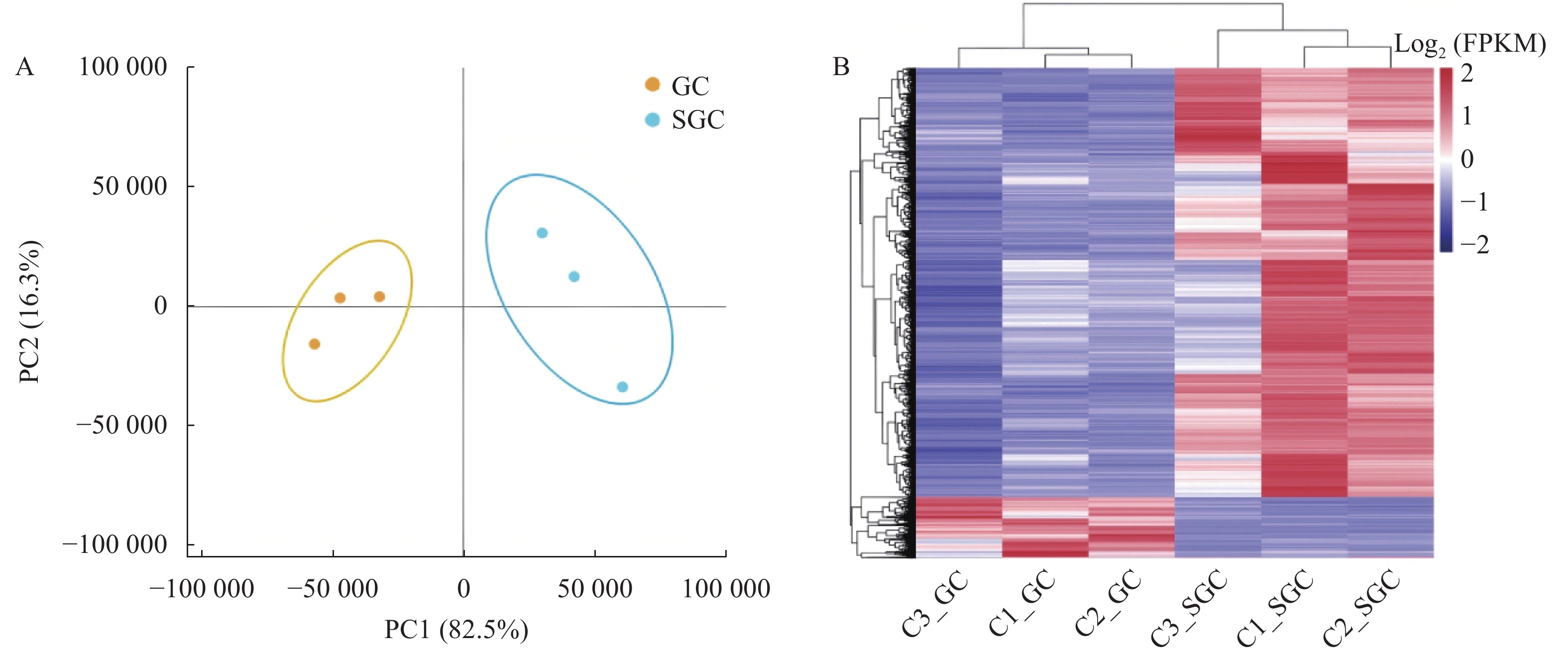
结果测序获得了红螯螯虾血细胞的非冗余唯一基因(unigene)共
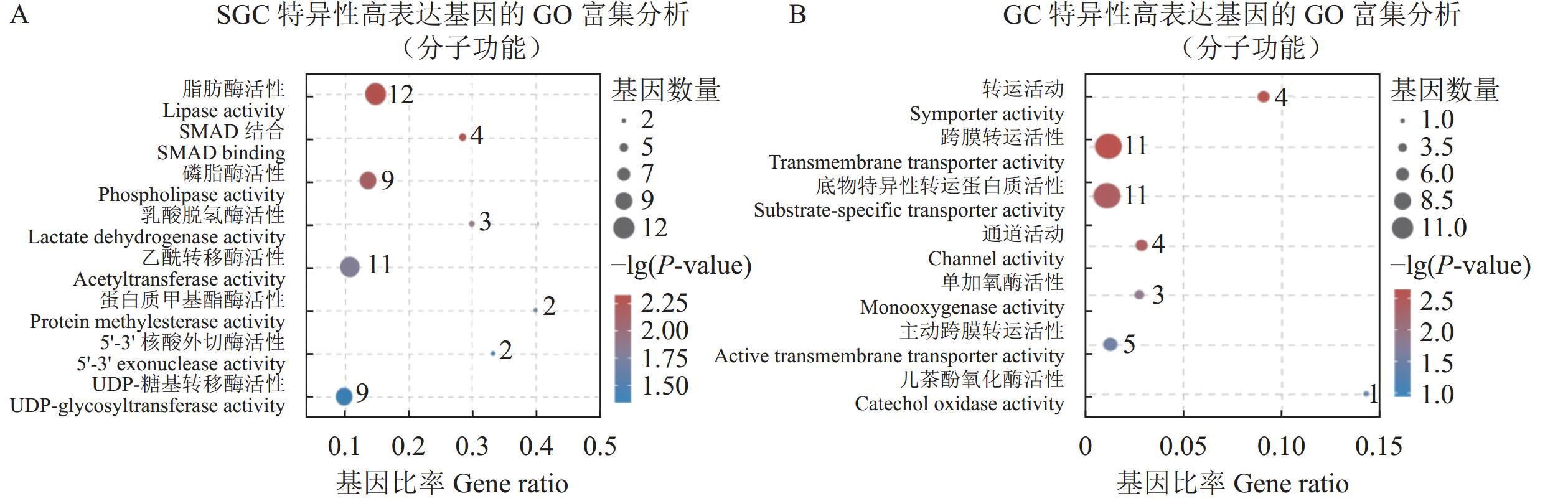
116199 个。这些unigenes的平均长度为763 bp,N50为1313 bp。经对比共发现了4488 个差异表达基因(differentially expressed genes, DEGs),其中3951 个基因在SGC中高度表达,537个基因在GC中高度表达。GO富集分析结果显示:在细胞组分类别中,SGC共富集到7个条目,GC共富集到10个条目;在分子功能类别中,SGC共富集到31个条目,GC共富集到60个条目;在生物过程类别中,SGC共富集到154个条目,GC共富集到102个条目。KEGG富集分析结果显示,SGC共富集到44条通路,GC共富集到10条通路。结论在SGC中高度表达的DEGs主要与细胞增殖、分化、基因表达调控、酶产生、内吞作用和细胞黏附有关;在GC中高度表达的DEGs主要与跨膜转运、代谢、酚氧化酶系统、吞噬作用和抗菌肽的产生有关。
Abstract:ObjectiveTranscriptomics of the two primary subpopulations of circulating hemocytes representing the distinct stages of immune cell differentiation in crayfish, semi-granular cell (SGC) and granular cell (GC), was studied.
MethodsSGC and GC in Cherax quadricarinatus were isolated and purified by means of Percoll discontinuous density gradient centrifugation. Transcriptome sequencing and analyses of differential expression, gene ontology (GO) enrichment, and Kyoto encyclopedia of genes and genomes (KEGG) enrichment on them were conducted. RT-qPCR was employed to validate the expressions of the differentially expressed genes (DEGs).
ResultsThe sequencing identified
116199 unigenes in the C. quadricarinatus hemocytes with an average length of 763 bp and an N50 of1313 bp. The4488 DEGs included3951 significantly upregulated in SGC and 537 in GC. On cellular components, there were 7 GO enrichment bands in SGC and 10 in GC; on molecular functions, 31 in SGC and 60 in GC; and on biological processes, 154 in SGC and 102 in GC. The KEGG analysis found 44 pathways enriched in SGC and 10 in GC.ConclusionThe DEGs enriched in SGC primarily involved in cellular proliferation, differentiation, transcriptional regulation, enzyme synthesis, endocytosis, and adhesion processes. Whereas those enriched in GC were basically associated with the transmembrane transport, metabolic pathways, prophenoloxidase system, phagocytosis, and antimicrobial peptide synthesis.
-
-
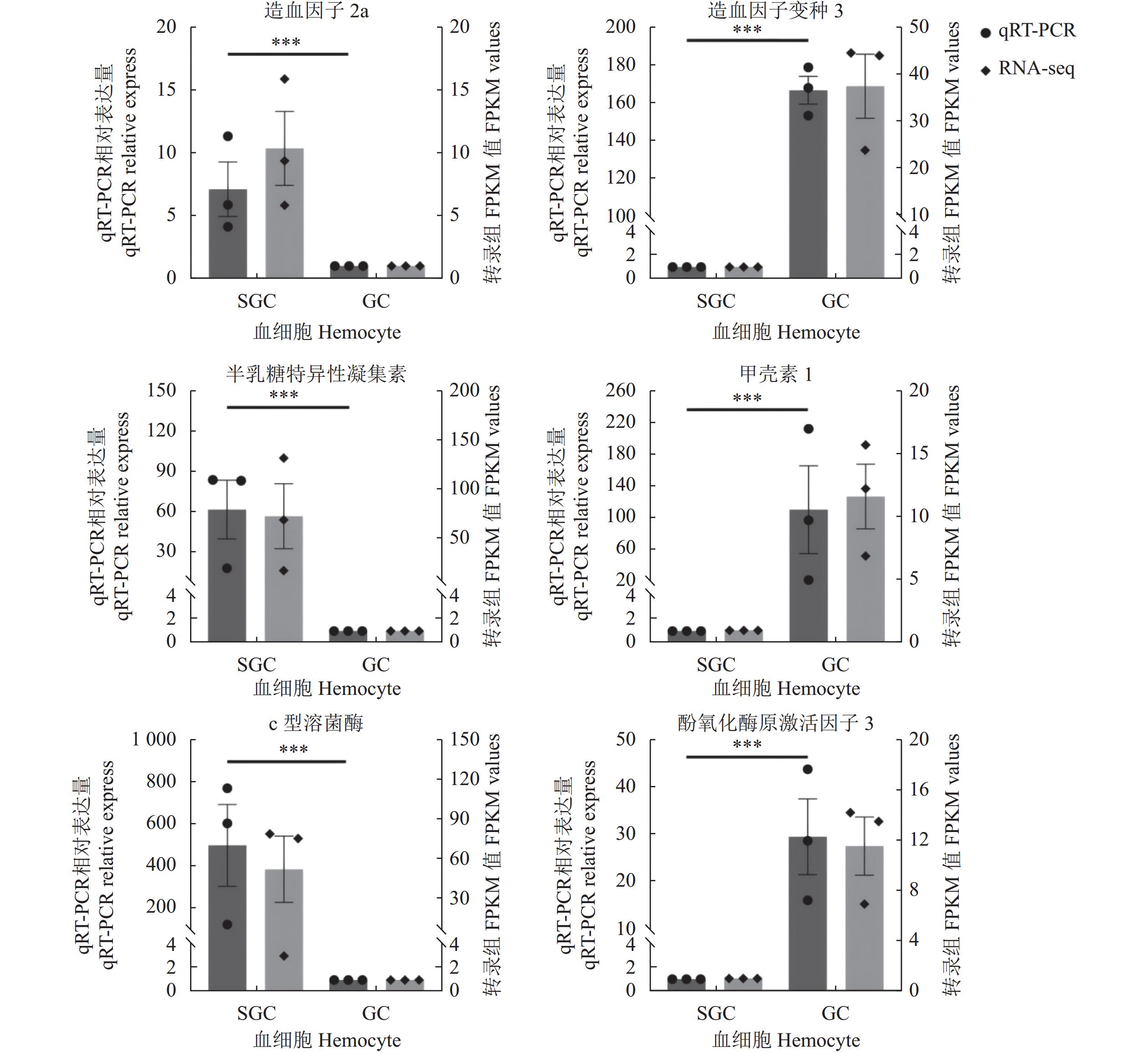
表 1 实时定量RT-PCR中使用的引物序列
Table 1 Sequences of primers for RT-qPCR
基因ID 注释Annotation 引物序列(5′-3′)
Primer sequence(5′-3′)Unigene0075897 c型溶菌酶 F:GTTAGCGTGCTCGTGGTTG R:TCCCGTAGACTTTTGCCG Unigene0027599 半乳糖特异性凝集素 F:ATTGGTGGGGCTGGAAGA R:TTTGAGTGTGATTGAGCAGAAGTA Unigene0049690 造血因子2a F:TAACCTTCACTACCCCAACAAT R:ACCAGTCCAGCTCCGCA Unigene0022931 酚氧化酶原激活因子3 F:GGGTTGATAATGCTTCCTTC R:TGCTTGTCTGGTCACTGGT Unigene0112798 造血因子变种3 F:CCAGTTGGCTGCCTCACA R:GACCACGACCGACTTTGC Unigene0006621 甲壳素1 F:TACAACACACTCGCAGCATCT R:GCAGTCTGGGGGGAACC 内参基因 Internal reference gene β-肌动蛋白 F:ATTACCATCCAGGCTGTGCT R:GGGCGAAACCTTCATACACG 表 2 基于FPKM值的两个血细胞亚群差异表达基因
Table 2 DEGs between SGC and GC based on FPKM value
基因ID 平均表达量 FPKM 注释 Annotation SGCs GCs Unigene0005146 5568.05 66.81 HPT因子9 HPT factor 9 [通讯鳌虾 Pacifastacus leniusculus] Unigene0034901 3514.74 50.20 NOTCH同源蛋白1 Predicted neurogenic locus notch homolog protein 1-like [美洲钩虾 Hyalella Azteca] Unigene0070406 3468.68 36.63 转谷氨酰胺酶 Transglutaminase [通讯鳌虾 Pacifastacus leniusculus] Unigene0043100 2227.69 43.31 钙蛋白酶B Calpain B [侧身地蟹 Gecarcinus lateralis] Unigene0081651 1817.96 32.81 半乳糖特异性凝集素(galactose-specific lectin nattectin-like protein, partial)[克氏原螯虾 Procambarus clarkii] Unigene0044871 1604.99 15.45 伸展蛋白结构域 Extensin-like [中国地鼠 Cricetulus griseus] Unigene0025602 986.96 12.41 锌指蛋白544样亚型X3 Zinc finger protein 544-like isoform X3 [双条吻蚓 Rhinatrema bivittatum] Unigene0049690 246.33 28.40 造血因子2a Astakine 2a [通讯螯虾 Pacifastacus leniusculus] Unigene0006621 1295.81 12639.05 甲壳素1 Crustin 1 [克氏原螯虾 Procambarus clarkii] Unigene0085460 1806.77 6228.55 酚氧化酶原 Prophenoloxidase [红螯螯虾 Cherax quadricarinatus] Unigene0106531 1065.41 3376.95 甲壳素2 Crustin 2 [红螯螯虾 Cherax quadricarinatus] Unigene0045189 265.39 2496.35 甘露糖结合蛋白 Mannose-binding protein [通讯螯虾 Pacifastacus leniusculus] Unigene0112798 53.04 1839.86 造血因子变种3 Astakine variant 3 [斑节对虾 Penaeus monodon] Unigene0013166 27.08 1283.46 细胞黏附蛋白 Peroxinectin [克氏原螯虾 Procambarus clarkii] -
[1] ROWLEY A F. The immune system of crustaceans[J]. Encyclopedia of Immunobiology,2016(1) :437−453.
[2] SöDERHäLL K. Invertebrate Immunity[M]. Boston,MA :Landes Bioscience and Springer Science and Business Media,LLC,2010:239–259.
[3] LIN X H,SÖDERHÄLL I. Crustacean hematopoiesis and the astakine cytokines[J]. Blood,2011,117(24) :6417−6424. DOI: 10.1182/blood-2010-11-320614
[4] VAN DE BRAAK C B T,BOTTERBLOM M H A,LIU W,et al. The role of the haematopoietic tissue in haemocyte production and maturation in the black tiger shrimp (Penaeus monodon) [J]. Fish & Shellfish Immunology,2002,12(3) :253−272.
[5] HOSE J E,MARTIN G G,GERARD A S. A decapod hemocyte classification scheme integrating morphology,cytochemistry,and function[J]. The Biological Bulletin,1990,178(1) :33−45. DOI: 10.2307/1541535
[6] LI F,ZHENG Z C,LI H Y,et al. Crayfish hemocytes develop along the granular cell lineage[J]. Scientific Reports,2021,11(1) :13099. DOI: 10.1038/s41598-021-92473-9
[7] 姚翠鸾,王志勇,相建海. 甲壳动物血细胞及其在免疫防御中的功能[J]. 动物学研究,2006,27(5) :549−557. DOI: 10.3321/j.issn:0254-5853.2006.05.014 YAO C L,WANG Z Y,XIANG J H. Crustacean haemocytes and their function in immune responses[J]. Zoological Research,2006,27(5) :549−557. (in Chinese) DOI: 10.3321/j.issn:0254-5853.2006.05.014
[8] PERSSON M,VEY A,SDERHLL K. Encapsulation of foreign particles in vitro by separated blood cells from crayfish,Astacus leptodactylus[J]. 1987,247(2) :409–415.
[9] 陈琪,康翠洁. 虾类血细胞的分类与功能研究进展[J]. 生物工程学报,2021,37(1) :53−66. CHEN Q,KANG C J. Advancements in the study of the classification and immune function of shrimp hemocytes[J]. Chinese Journal of Biotechnology,2021,37(1) :53−66. (in Chinese)
[10] SRICHAROEN S,KIM J J,TUNKIJJANUKIJ S,et al. Exocytosis and proteomic analysis of the vesicle content of granular hemocytes from a crayfish[J]. Developmental & Comparative Immunology,2005,29(12) :1017−1031.
[11] LI F,CHANG X F,XU L M,et al. Different roles of crayfish hemocytes in the uptake of foreign particles[J]. Fish & Shellfish Immunology,2018,77:112−119.
[12] WEI C,PAN L Q,ZHANG X,et al. Transcriptome analysis of hemocytes from the white shrimp Litopenaeus vannamei with the injection of dopamine[J]. Fish & Shellfish Immunology,2019,94:497−509.
[13] HE Z H,ZHAO J C,CHEN X Y,et al. The molecular mechanism of hemocyte immune response in Marsupenaeus japonicus infected with decapod iridescent virus 1[J]. Frontiers in Microbiology,2021,12:710845. DOI: 10.3389/fmicb.2021.710845
[14] CHENG C H,MA H L,DENG Y Q,et al. Effects of Vibrio parahaemolyticus infection on physiological response,histopathology and transcriptome changes in the mud crab (Scylla paramamosain) [J]. Fish & Shellfish Immunology,2020,106:197−204.
[15] CHEN D F,LU L,PEI Q L,et al. Transcriptome analysis of the immunomodulatory effects of Salvia miltiorrhiza polysaccharide on hemocyte immune response in Procambarus clarkii[J]. Fish & Shellfish Immunology,2022,131:697−706.
[16] LIAO X Z,WANG C G,WANG B,et al. Research into the hemocyte immune response of Fenneropenaeus merguiensis under decapod iridescent virus 1 (DIV1) challenge using transcriptome analysis[J]. Fish & Shellfish Immunology,2020,104:8−17.
[17] 傅一鸣,李智,柳峰松,等. 微囊藻毒素MC-LR对凡纳滨对虾细胞免疫相关基因表达水平的影响[J]. 上海海洋大学学报,2015,24(2) :196−202. FU Y M,LI Z,LIU F S,et al. The toxicity impact of microcystin on expression of cellular immune-related genes in Litopenaeus vannamei[J]. Journal of Shanghai Ocean University,2015,24(2) :196−202. (in Chinese)
[18] 傅蓉蓉,李钫,杨丰. Percoll不连续密度梯度离心法分离红螯光壳螯虾血细胞[J]. 水产学报,2019,43(4) :841−851. FU R R,LI F,YANG F. Separation of hemocytes of Cherax quadricarinatus by Percoll discontinuous density gradient centrifugation[J]. Journal of Fisheries of China,2019,43(4) :841−851. (in Chinese)
[19] GRABHERR M G,HAAS B J,YASSOUR M,et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome[J]. Nature Biotechnology,2011,29(7) :644−652. DOI: 10.1038/nbt.1883
[20] LI B,DEWEY C N. RSEM:Accurate transcript quantification from RNA-Seq data with or without a reference genome[J]. BMC Bioinformatics,2011,12:323. DOI: 10.1186/1471-2105-12-323
[21] MORTAZAVI A,WILLIAMS B A,MCCUE K,et al. Mapping and quantifying mammalian transcriptomes by RNA-seq[J]. Nature Methods,2008,5(7) :621−628. DOI: 10.1038/nmeth.1226
[22] LIVAK K J,SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T) ) Method[J]. Methods,2001,25(4) :402−408. DOI: 10.1006/meth.2001.1262
[23] SIMÃO F A,WATERHOUSE R M,IOANNIDIS P,et al. BUSCO:Assessing genome assembly and annotation completeness with single-copy orthologs[J]. Bioinformatics,2015,31(19) :3210−3212. DOI: 10.1093/bioinformatics/btv351
[24] HALL M,WANG R,VAN ANTWERPEN R,et al. The crayfish plasma clotting protein:A vitellogenin-related protein responsible for clot formation in crustacean blood[J]. Proceedings of the National Academy of Sciences of the United States of America,1999,96(5) :1965−1970.
[25] MANINGAS M B B,KONDO H,HIRONO I,et al. Essential function of transglutaminase and clotting protein in shrimp immunity[J]. Molecular Immunology,2008,45(5) :1269−1275. DOI: 10.1016/j.molimm.2007.09.016
[26] LIN X H,SÖDERHÄLL K,SÖDERHÄLL I. Transglutaminase activity in the hematopoietic tissue of a crustacean,Pacifastacus leniusculus,importance in hemocyte homeostasis[J]. BMC Immunology,2008,9:58. DOI: 10.1186/1471-2172-9-58
[27] LOPES-FERREIRA M,MAGALHÃES G S,FERNANDEZ J H,et al. Structural and biological characterization of Nattectin,a new C-type lectin from the venomous fish Thalassophryne nattereri[J]. Biochimie,2011,93(6) :971−980. DOI: 10.1016/j.biochi.2011.03.001
[28] JIANG H S,JIA W M,ZHAO X F,et al. Four crustins involved in antibacterial responses in Marsupenaeus japonicus[J]. Fish & Shellfish Immunology,2015,43(2) :387−395.
[29] SÁNCHEZ-SALGADO J L,PEREYRA M A,ALPUCHE-OSORNO J J,et al. Pattern recognition receptors in the crustacean immune response against bacterial infections[J]. Aquaculture,2021,532:735998. DOI: 10.1016/j.aquaculture.2020.735998
[30] HOLMBLAD T,SÖDERHÄLL K. Cell adhesion molecules and antioxidative enzymes in a crustacean,possible role in immunity[J]. Aquaculture,1999,172(1/2) :111−123.
[31] LV S J,LU B J,XU J H,et al. Immune response of peroxinectin of Chinese mitten crab Eriocheir sinensis to exterior stimulation[J]. Developmental & Comparative Immunology,2015,51(1) :56−64.
[32] SUN M Z,LI S H,ZHANG X J,et al. Isolation and transcriptome analysis of three subpopulations of shrimp hemocytes reveals the underlying mechanism of their immune functions[J]. Developmental & Comparative Immunology,2020,108:103689.
[33] SÖDERHÄLL I,JUNKUNLO K. A comparative global proteomic analysis of the hematopoietic lineages in the crustacean Pacifastacus leniusculus[J]. Developmental & Comparative Immunology,2019,92:170−178.
[34] MIYAZAWA K,MIYAZONO K. Regulation of TGF-β family signaling by inhibitory smads[J]. Cold Spring Harbor Perspectives in Biology,2017,9(3) :a022095. DOI: 10.1101/cshperspect.a022095
[35] ZHAO M,MISHRA L,DENG C X. The role of TGF-β/SMAD4 signaling in cancer[J]. International Journal of Biological Sciences,2018,14(2) :111−123. DOI: 10.7150/ijbs.23230
[36] LAI L Y S,GRACIE N P,GOWRIPALAN A,et al. SMAD proteins:Mediators of diverse outcomes during infection[J]. European Journal of Cell Biology,2022,101(2) :151204. DOI: 10.1016/j.ejcb.2022.151204
[37] KOIWAI K,KOYAMA T,TSUDA S,et al. Single-cell RNA-seq analysis reveals penaeid shrimp hemocyte subpopulations and cell differentiation process[J]. eLife,2021,10:e66954. DOI: 10.7554/eLife.66954
[38] CUI C,TANG X Q,XING J,et al. Single-cell RNA-seq uncovered hemocyte functional subtypes and their differentiational characteristics and connectivity with morphological subpopulations in Litopenaeus vannamei[J]. Frontiers in Immunology,2022,13:980021. DOI: 10.3389/fimmu.2022.980021
[39] CERENIUS L,LEE B L,SÖDERHÄLL K. The proPO-system:Pros and cons for its role in invertebrate immunity[J]. Trends in Immunology,2008,29(6) :263−271. DOI: 10.1016/j.it.2008.02.009
[40] WEISS H J,ANGIARI S. Metabolite transporters as regulators of immunity[J]. Metabolites,2020,10(10) :418. DOI: 10.3390/metabo10100418
[41] DUAN H,JIN S J,ZHANG Y,et al. Granulocytes of the red claw crayfish Cherax quadricarinatus can endocytose beads,E. coli and WSSV,but in different ways[J]. Developmental & Comparative Immunology,2014,46(2) :186−193.
[42] LIU S,ZHENG S C,LI Y L,et al. Hemocyte-mediated phagocytosis in crustaceans[J]. Frontiers in Immunology,2020,11:268.
[43] BENTON J L,KERY R,LI J J,et al. Cells from the immune system generate adult-born neurons in crayfish[J]. Developmental Cell,2014,30(3) :322−333. DOI: 10.1016/j.devcel.2014.06.016
[44] BELTZ B S,BRENNEIS G,BENTON J L. Adult neurogenesis:Lessons from crayfish and the elephant in the room[J]. Brain,Behavior and Evolution,2016,87(3) :146−155.
[45] BELTZ B S,BENTON J L. From blood to brain:Adult-born neurons in the crayfish brain are the progeny of cells generated by the immune system[J]. Frontiers in Neuroscience,2017,11:662.
[46] CHAVES DA SILVA P G,BENTON J L,SANDEMAN D C,et al. Adult neurogenesis in the crayfish brain:The hematopoietic anterior proliferation center has direct access to the brain and stem cell niche[J]. Stem Cells and Development,2013,22(7) :1027−1041.
[47] NOONIN C,LIN X H,JIRAVANICHPAISAL P,et al. Invertebrate hematopoiesis:An anterior proliferation center as a link between the hematopoietic tissue and the brain[J]. Stem Cells and Development,2012,21(17) :3173−3186.




 下载:
下载: