Expression of BMPs in Developing Chicken Embryos
-
摘要:目的 分析骨形成蛋白(Bone morphogenetic proteins,BMPs)基因在鸡胚不同发育阶段的表达水平,为进一步研究BMPs基因的功能提供依据。方法 选取AA肉鸡种蛋100枚进行孵化,分别于孵化期第1、2、3、4、5、6、9、12、15、18 d(记为E1~E18)选取胚蛋6枚,E1~E6采集整胚,E12~E18分别采集大脑、心脏、肝脏和腿肌组织。采用定量PCR(qPCR)方法检测BMP2、BMP4和BMP7基因的表达丰度。结果 BMP2基因在E1~E6中的表达水平呈现先上升后下降的趋势;BMP2在E4、E5和E6中的表达水平显著高于E1(P<0.05);BMP2在大脑中的表达显示,E12显著高于E1(P<0.05),而到了E18阶段反而极显著低于E1(P<0.01);BMP2在心脏和腿肌中的表达均是E9显著或极显著大于E1(P<0.05或P<0.01);鸡胚发育到了后期(E15和E18),BMP2在心脏和肝脏中的表达却显著或极显著低于E1时期(P<0.05或P<0.01)。BMP4在E1~E6中的表达水平也是呈现先上升后下降的趋势;BMP4基因在E3~E6整胚和E9胚龄的大脑、心脏、肝脏和腿肌中的表达水平均显著或极显著高于E1胚龄(P<0.05或P<0.01)。BMP7在E4、E5和E6的表达与BMP2、BMP4类似,即BMP7基因的表达水平显著或极显著高于E1时期(P<0.05或P<0.01),但在E2和E3时期,BMP7表达水平反而降低了,且E2时期极显著低于E1时期(P<0.01);在整胚和大脑中的结果显示,E4~E18阶段BMP7基因的表达水平呈现逐渐降低趋势,到E18时期大脑中的BMP7基因的表达水平极显著低于E1时期(P<0.01);与E1相比,在心脏中BMP7到E12时期达到最低水平(P<0.01),而肝脏中BMP7基因表达水平在E12和E15阶段均极显著低于E1(P<0.01)。结论 BMP2、BMP4和BMP7基因在整胚的表达水平呈现先上升后下降趋势,至E4时期到达最高点;BMP2基因在E9、E12、E15和E18的心脏、肝脏和腿肌中的表达均呈现下降趋势。说明BMPs基因在器官形成初期起着关键作用,之后随着器官发育完成BMPs基因的作用逐渐减弱。Abstract:Objective Expression of bone morphogenetic proteins (BMPs) gene in chicken embryos at different developmental stages was studied for further investigation on the functions of the gene.Method One hundred fertilized AA broiler eggs were used for the study. Six eggs were randomly selected each time on the 1st, 2nd, 3rd, 4th, 5th, 6th, 9th, 12th, 15th, and 18th day (designated as E1 to E18) after hatching. Whole embryos from the eggs of E1 to E6 and the brain, heart, and liver tissues as well as the leg muscles from the embryos of E12 to E18 were collected to determine the expressions of BMP2, BMP4 and BMP7 in the samples by qPCR.Result The BMP2 expressions of E1–E6 increased at first and then decreased. Those of E4, E5, and E6 significantly higher than that of E1 (P<0.05). BMP2 in the brain of E12 was significantly higher than that of E1 (P<0.05), but that of E18 became significantly lower than that of E1 (P<0.01). For E9, the expression of BMP2 in the heart was significantly higher than that for E1 at P<0.05 and for the leg muscles at P<0.01. In the later stages of embryonic development, such as E15 and E18, the expressions in the heart and liver were significantly or extremely significantly lower than those of E1 at P<0.05 or P<0.01. The expression of BMP4 of E1–E6 also increased initially and followed by a decline. Compared to E1, E3–E6 in embryo and E9 in brain, heart, liver and leg showed significantly higher on BMP4 expressions at P<0.05 or at P<0.01. BMP7 of E4 expressed significantly higher than E1 at P<0.01, and of E5 and E6 at P<0.05, but lower of E2 or E3 with a statistic significance at P<0.01 on E2. From E4 through E18, the BMP7 expressions in the embryo and brain declined gradually, and that in the brain reached a significant level at P<0.01 on E18. In the heart, the lowest expression was observed on E12 (P<0.01), and in the liver on E12 and E15 (P<0.01).Conclusion As hatching progressed, the expressions of BMP2, BMP4 and BMP7 in the embryo firstly increased then decreased to arrive at a peak on E4, while BMP2 in the heart, liver and leg decreased on E9, E12, E15 and E18. It indicated that the BMP genes played a crucial role in the early stages of organ formation in fertilized chicken eggs. The effect diminished gradually as the embryonic development came close to completion.
-
Keywords:
- broiler /
- bone morphogenetic proteins /
- chicken embryo /
- expression
-
0. 引言
【研究意义】胚胎期是动物生长发育的关键时期,此时机体生长发育的状况对动物出生后的各项性能具有极其重要的影响。由于鸡胚具有发育周期短、易于操作等特点,所以鸡胚成为研究胚胎发育和器官形成机制的最佳模型[1]。另外,鸡胚发育过程受到多种基因严格调控,保证胚胎有序地进行组织分化和个体发育。大脑、心脏和肝脏是鸡胚发育过程中较早发育的重要器官。因此,以鸡胚为试验材料,研究骨形成蛋白(Bone morphogenetic proteins, BMPs)基因在鸡胚中的表达规律,可为进一步研究BMPs基因的功能提供依据。【前人研究进展】BMPs蛋白是转化生长因子β(transforming growth factor-β, TGFβ)超家族的多功能生长因子,它能够诱导动物或人体间充质细胞分化为骨、软骨、韧带、肌腱和神经组织[2-3];BMPs不但能促进骨骼形成并帮助修复骨折,而且可以通过抑制肌肉生成来指导体细胞的发育[3]。目前,BMPs在胚胎发育、动物出生后以及成年动物细胞功能中的作用越来越受到重视。最初,BMPs按照氨基酸序列及同源性分为BMP1~7种,但随着研究的深入,现已发现20多种BMPs[3-4]。已证明BMP2、4、6、7、9具有显著的成骨特性[5-6]。BMP2在诱发软骨及硬骨的生成、成骨细胞的分化扮演重要角色[7-9];BMP4可以调节牙齿、四肢以及由中胚层发育而来的硬骨形成,其对骨折的修复、上表皮的形成、腹背轴的形成,以及卵巢滤泡的发育过程发挥作用[3, 10-11];BMP7主要在成骨细胞的分化、脂肪形成和能量消耗、肾的发育及修复中有重要作用[12-14]。【本研究切入点】目前,尚未见有BMP2、BMP4、BMP7基因在鸡胚中定量表达研究的报道。【拟解决的关键问题】本文研究BMP2、BMP4、BMP7基因在鸡胚不同发育阶段的表达水平,以期为BMPs基因功能研究奠定基础。
1. 材料与方法
1.1 鸡胚孵化与样品采集
选取新鲜的艾拔益加(Arbor Acres, AA)肉鸡种蛋100枚(蛋重50~55 g),洗净,用高锰酸钾熏蒸消毒后放入温度为(37±0.5)℃、湿度为60 %的孵化箱中孵化,每隔2 h自动翻蛋1次。分别于孵化期第1、2、3、4、5、6、9、12、15、18 d(记为E1、E2、E3、E4、E5、E6、E9、E12、E15、E18)同一时间选取大小一致、发育良好的胚蛋6枚,E1~E6采集整胚,E12~E18分别采集大脑、心脏、肝脏和左侧大腿肌肉组织放入冻存管液氮速冻,然后转入−80℃冰箱备用。
1.2 总RNA提取与cDNA的合成
采用Trizol(Invitrogen公司)法从整胚、脑、心、肝和腿部肌肉中提取总RNA。用核酸蛋白检测仪检测总RNA的纯度和浓度。利用M-MLV(Promega公司)反转录酶试剂盒,按照说明书合成cDNA第一链。
1.3 引物设计与荧光定量PCR扩增(qPCR)
根据GenBank上鸡的BMP2(登录号:NM_204358)、BMP4(登录号:NM_205237)和BMP7(登录号:AF205877)基因序列,利用Primer Premier 6.0软件设计qPCR引物。以鸡GAPDH(登录号:NM_204305)基因为内参,引物序列由北京鼎国生物技术有限责任公司合成(表1)。
表 1 qPCR引物序列、扩增片段大小和退火温度Table 1. Primer sequences, product sizes and annealing temperature applied for qPCR analysis基因 Gene 引物序列(5′→3′)Primer sequence (5′→3′) 片段大小 Amplicon/bp 退火温度 Annealing temperature /℃ 登录号 Accession No. BMP2 TGGTGGAGGTGGTTCACTT 181 60 NM_204358 TTGTGTTTCGCTTGACGC BMP4 CTTCGTCTTCAACCTCAGC 150 59 NM_205237 ACAGCGGCTTCATCACTT BMP7 CTGATTTGTTCCTGCTCG 152 58 AF205877 GCTTTGCCCATCTATGCT GAPDH CTACACACGGACACTTCAAG 244 59 NM_204305 ACAAACATGGGGGCATCAG 采用SYBR Green I(Tiangen公司)染料法,以鸡GAPDH基因为内参,进行BMP2、BMP4和BMP7基因qPCR分析。qPCR反应组成:FastFire qPCR PreMix 10 μL,引物各0.2 μL,cDNA(稀释10倍)1 μL,灭菌超纯水加至20 μL。qPCR反应程序:95℃ 5 min;95℃ 20 s,退火温度(表1)10 s,72℃ 1 s,重复40个循环。每个循环后采集荧光生成扩增曲线。为了分析qPCR扩增的特异性,温度从58℃缓慢升温到95℃,连续测定样品的荧光强度以获取熔解曲线。所有样本进行3个重复测定,并在每次试验时设阴性对照。根据荧光曲线的Ct值及标准曲线,以鸡GAPDH基因为内参,用2−△△Ct法计算BMP2、BMP4和BMP7基因mRNA的相对表达量。
1.4 统计分析
BMP2、BMP4和BMP7基因的mRNA表达水平表示为平均值±标准差(SD)。利用SAS 8.2软件,采用单因子方差分析(One-way ANOVA)分析平均数间的差异显著性。当P<0.05时,差异具有统计学意义。
2. 结果与分析
2.1 BMP2基因的表达水平
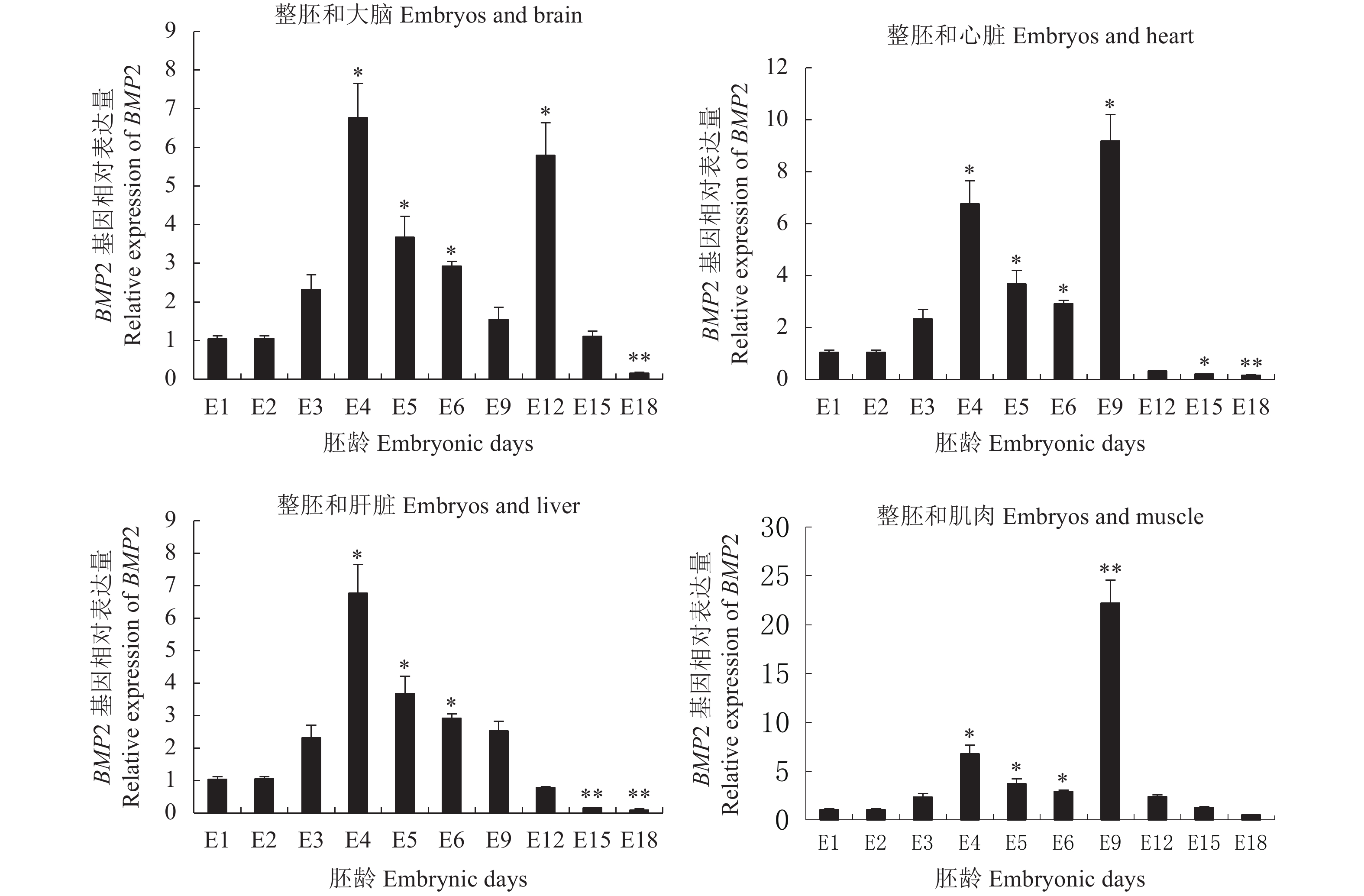
以不同发育阶段的鸡胚cDNA为模板,进行BMP2基因qPCR扩增,试验结果如图1所示。从图1可见,BMP2基因在整胚(E1~E6)中的表达水平呈现先上升后下降趋势;与E1相比,BMP2基因在E4、E5和E6中的表达水平显著增高(P<0.05)。BMP2在大脑中的表达显示E12时期显著高于E1时期(P<0.05),而到了E18阶段反而极显著低于E1阶段(P<0.01)。BMP2在心脏和腿肌中的表达E9阶段显著或极显著大于E1时期(P<0.05或P<0.01);鸡胚发育到了后期(E15和E18),BMP2基因在心脏和肝脏中的表达却显著或极显著低于E1时期(P<0.05或P<0.01)。
![]() 图 1 BMP2基因的表达水平注:图1表示BMP2基因在E1~E6整胚和E9~E18胚龄大脑、心脏、肝脏和腿肌中的表达水平(平均值±标准差)(n=6)。*或**表示BMP2基因在E2~E18胚龄中的表达水平与E1中的表达水平相比差异显著(P<0.05)或极显著(P<0.01)。Figure 1. Expression of BMP2Note: It represent expression levels of BMP2 gene in whole embryos of E1–E6 and in brain, heart, liver and muscle of E9–E18 (Mean ± SD) (n=6), respectively. * or ** indicates significant difference between E1 and E2–E18 (P<0.05 or P<0.01).
图 1 BMP2基因的表达水平注:图1表示BMP2基因在E1~E6整胚和E9~E18胚龄大脑、心脏、肝脏和腿肌中的表达水平(平均值±标准差)(n=6)。*或**表示BMP2基因在E2~E18胚龄中的表达水平与E1中的表达水平相比差异显著(P<0.05)或极显著(P<0.01)。Figure 1. Expression of BMP2Note: It represent expression levels of BMP2 gene in whole embryos of E1–E6 and in brain, heart, liver and muscle of E9–E18 (Mean ± SD) (n=6), respectively. * or ** indicates significant difference between E1 and E2–E18 (P<0.05 or P<0.01).2.2 BMP4基因的表达水平
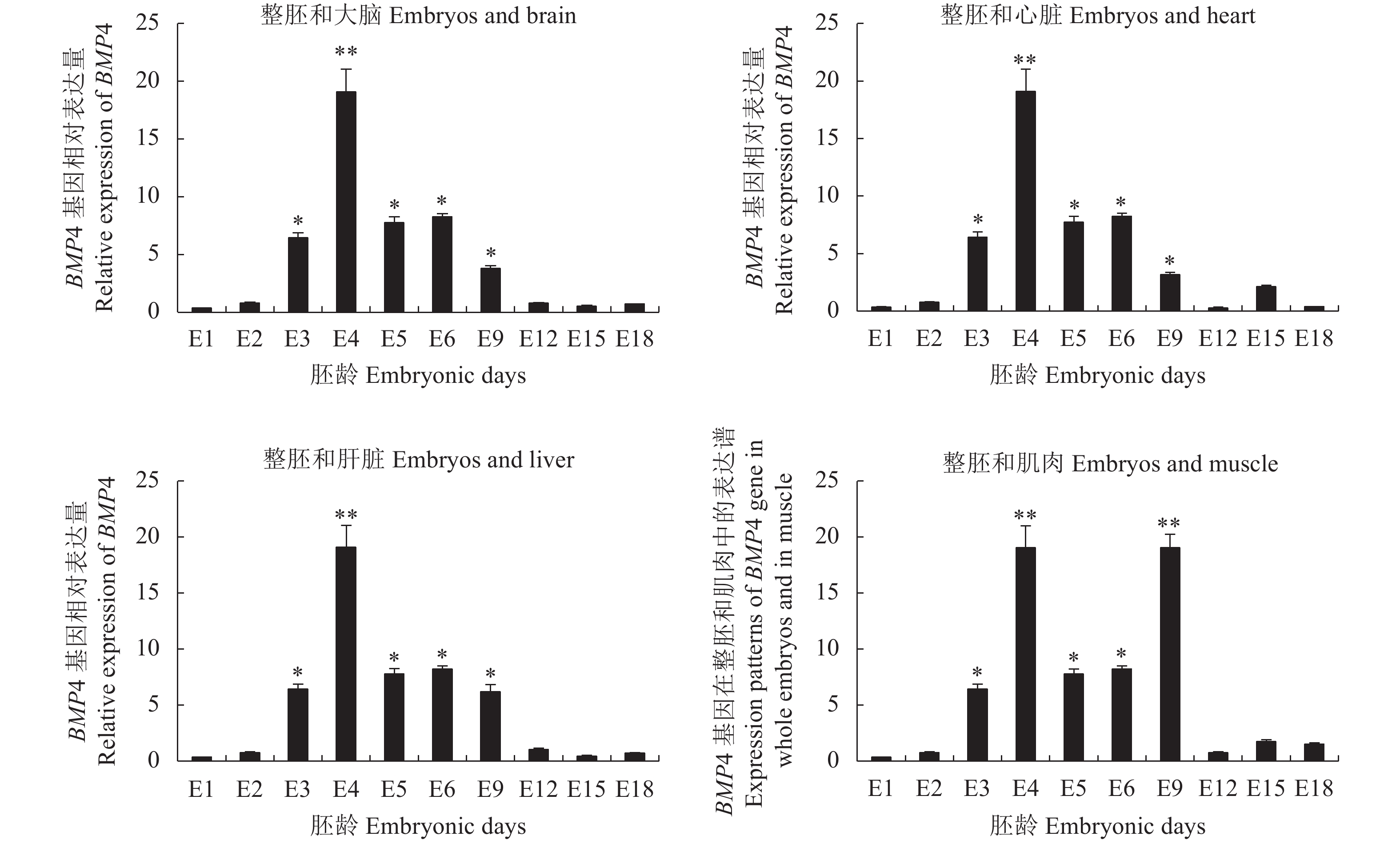
BMP4基因表达水平如图2所示。与BMP2类似,BMP4基因在整胚(E1~E6)中的表达水平也是呈现先上升后下降的变化趋势;与E1相比,BMP4基因在E3~E6整胚和E9胚龄的大脑、心脏、肝脏和腿肌中的表达水平均显著或极显著增加(P<0.05或P<0.01);而在E2、E12、E15和E18胚龄时期,BMP4基因的表达与E1相比均未达到差异显著水平(P>0.05)。
![]() 图 2 BMP4基因的表达水平注:图2表示BMP4基因在E1~E6整胚和E9~E18胚龄大脑、心脏、肝脏和腿肌中的表达水平(平均值±标准差)(n=6)。*或**表示BMP4基因在E2~E18胚龄中的表达水平与E1中的表达水平相比差异显著(P<0.05)或极显著(P<0.01)。Figure 2. Expression of BMP4Note: It represent expression levels of BMP4 gene in whole embryos of E1–E6 and in brain, heart, liver and muscle of E9–E18 (Mean ± SD) (n=6), respectively. * or ** indicates significant difference between E1 and E2–E18 (P<0.05 or P<0.01).
图 2 BMP4基因的表达水平注:图2表示BMP4基因在E1~E6整胚和E9~E18胚龄大脑、心脏、肝脏和腿肌中的表达水平(平均值±标准差)(n=6)。*或**表示BMP4基因在E2~E18胚龄中的表达水平与E1中的表达水平相比差异显著(P<0.05)或极显著(P<0.01)。Figure 2. Expression of BMP4Note: It represent expression levels of BMP4 gene in whole embryos of E1–E6 and in brain, heart, liver and muscle of E9–E18 (Mean ± SD) (n=6), respectively. * or ** indicates significant difference between E1 and E2–E18 (P<0.05 or P<0.01).2.3 BMP7基因的表达水平
图3为BMP7基因在鸡胚中的表达水平。结果表明,E4、E5和E6时期BMP7 基因表达与BMP2、BMP4类似,即BMP7基因的表达水平显著或极显著高于E1时期(P<0.05或P<0.01),但在E2和E3时期,BMP7基因表达水平降低,且E2时期极显著低于E1时期(P<0.01)。在整胚和大脑中的结果显示,从E4~E18阶段BMP7基因的表达水平呈现逐渐降低趋势,到E18时期极显著低于E1时期(P<0.01)。在心脏、肝脏和腿肌中也出现类似的情况。相较于E1,心脏部位的BMP7表达在E12阶段达到最低水平(P<0.01),而肝脏中BMP7基因水平到E12和E15阶段均极显著低于E1阶段(P<0.01)。BMP7基因在其余组织和胚龄中的表达均未达到差异显著水平(P>0.05)。
![]() 图 3 BMP7基因的表达水平注:图3表示BMP7基因在E1~E6整胚和E9~E18胚龄大脑、心脏、肝脏和腿肌中的表达水平(平均值±标准差)(n=6)。*或**表示BMP7基因在E2~E18胚龄中的表达水平与E1中的表达水平相比差异显著(P<0.05)或极显著(P<0.01)。Figure 3. Expression of BMP7Note: It represent expression levels of BMP7 gene in whole embryos of E1–E6 and in brain, heart, liver and muscle of E9–E18 (Mean ± SD) (n=6), respectively. * or ** indicates significant difference between E1 and E2–E18 (P<0.05 or P<0.01).
图 3 BMP7基因的表达水平注:图3表示BMP7基因在E1~E6整胚和E9~E18胚龄大脑、心脏、肝脏和腿肌中的表达水平(平均值±标准差)(n=6)。*或**表示BMP7基因在E2~E18胚龄中的表达水平与E1中的表达水平相比差异显著(P<0.05)或极显著(P<0.01)。Figure 3. Expression of BMP7Note: It represent expression levels of BMP7 gene in whole embryos of E1–E6 and in brain, heart, liver and muscle of E9–E18 (Mean ± SD) (n=6), respectively. * or ** indicates significant difference between E1 and E2–E18 (P<0.05 or P<0.01).3. 讨论与结论
最近研究发现,BMPs不但具有促进骨生长和成骨细胞分化的特性,而且与细胞增殖以及分化、凋亡、形态发生时的细胞通讯等细胞发育过程有直接或间接关系[8-9, 14]。本研究以不同发育阶段鸡胚为研究对象,应用qPCR方法分析了BMP2、BMP4、BMP7基因在整胚和肌肉、心脏、肝脏和大脑中的表达水平变化。结果表明,BMP2和BMP4在E1~E6中的表达均呈现先上升后下降趋势;BMP7基因在E4~E6中的表达呈现下降趋势;BMP2、BMP4、BMP7基因在E9以后不同组织中表达水平呈现不同的变化趋势;与E1相比,BMPs基因在E9~E18时期4种组织的表达量存在一定差异,反应了BMPs基因的表达有一定的时空规律性。值得注意的是,BMPs基因在整胚中的表达均在E4时到达最高点,推测这个时期BMPs基因的高表达可能对器官的形成起着重要作用,因为E4时期机体的器官均开始发育,胚胎头部明显增大,是器官形成的关键时期。另外,研究表明BMPs是鸡胚前心外膜特性的重要调节因子,BMPs的时空表达模式对鸡胚的心肌形成至关重要[15-16],并且发现BMPs基因在鸡胚顶外胚层嵴有较强的表达[17]。以上研究结果为进一步研究BMPs基因的功能和信号传导途径提供了资料。
众所周知,BMP2在骨折愈合中起着重要作用,BMP2基因的表达是启动骨折愈合和调节胚胎发育的必要条件[18-19],所以,BMP2是脊椎动物发育不可缺少的多功能调节器[2, 20]。因此,BMP2在与骨形成、体内平衡和再生相关的生物学过程中起着至关重要的作用[21]。在动物上的研究表明,BMP2基因在母羊卵巢中的表达增强可导致体外雌二醇浓度升高[22],而BMP2基因在绵羊体内的表达可抑制孕酮浓度[23];在鸡的研究上也检测到非闭锁性窦状卵泡膜和颗粒中BMP2的表达[24]。最近发现,BMP2基因在小尾寒羊下丘脑、输卵管、卵巢、垂体和小脑中的相对表达高于苏尼特羊[25]。本研究发现BMP2在E9~E18阶段4种组织的表达除了在E12阶段大脑组织有所上升外,BMP2在其余3种组织的表达均呈现下降趋势。
BMP4不但在骨骼形成和骨折修复中发挥重要作用,而且与肌肉形成、卵母细胞成熟、卵泡发育和胚胎发育等密切相关[24, 26-30]。据报道,BMP4在斑马鱼的嗅鞘、眼、囊泡、心脏、生殖管、肛门、肠道、胸鳍和尾鳍芽中表达[26],而对鲤鱼和罗非鱼研究结果表明,BMP4基因在不同部位肌肉中的表达存在差异[29]。对鸡和鹅的研究发现,BMP4的相对丰度随着卵泡发育过程不断增加,在F5卵泡中达到最大值[28, 30]。本研究中,BMP4在4种组织中的相对丰度均是在E9阶段最高,在E12~E18阶段均呈不规则的变化。
BMP7广泛表达于人类胸腺、骨髓、脾脏、大脑、脊髓、心脏、骨骼肌、肾脏、肺、肝、胰腺和前列腺,对骨骼修复和再生、肾脏和眼睛形成、神经系统发育有重要作用[31-32]。在绵羊上的研究发现,小尾寒羊脑垂体、脑、下丘脑、输卵管和卵巢中BMP7基因的表达明显高于苏尼特羊[24]。据报道,BMP7能诱导人骨髓间充质干细胞向脂肪细胞分化和解偶联蛋白1(uncoupling protein 1, UCP1)表达[33]。最近研究表明,BMP7基因能调控鸡卵巢功能和黑色素生成,对蓝壳鸡的鸡冠色素沉着和产卵具有多效性[34]。本研究中,在鸡胚E9~E18发育阶段BMP7基因在大脑、心脏和肌肉中的表达量均是在E9阶段最高,且在大脑中的表达呈现下降趋势,在其他3种组织中均是不规则变化。
目前,对BMPs的研究越来越深入,一些新的功能也被发现。比如,BMP2和BMP4能促进白色脂肪发育,而BMP7与棕色脂肪发育有关[13, 35]。不但BMP2能增加雌二醇的产生,提高卵巢颗粒细胞的存活率,而且BMP7也有同样的功能[36]。另外,BMP2和BMP7可能还是影响绵羊产仔数的候选基因[37-38]。因此,随着研究的不断深入,BMPs的更多功能将被挖掘,为进一步改善动物遗传性状奠定基础。
-
图 1 BMP2基因的表达水平
注:图1表示BMP2基因在E1~E6整胚和E9~E18胚龄大脑、心脏、肝脏和腿肌中的表达水平(平均值±标准差)(n=6)。*或**表示BMP2基因在E2~E18胚龄中的表达水平与E1中的表达水平相比差异显著(P<0.05)或极显著(P<0.01)。
Figure 1. Expression of BMP2
Note: It represent expression levels of BMP2 gene in whole embryos of E1–E6 and in brain, heart, liver and muscle of E9–E18 (Mean ± SD) (n=6), respectively. * or ** indicates significant difference between E1 and E2–E18 (P<0.05 or P<0.01).
图 2 BMP4基因的表达水平
注:图2表示BMP4基因在E1~E6整胚和E9~E18胚龄大脑、心脏、肝脏和腿肌中的表达水平(平均值±标准差)(n=6)。*或**表示BMP4基因在E2~E18胚龄中的表达水平与E1中的表达水平相比差异显著(P<0.05)或极显著(P<0.01)。
Figure 2. Expression of BMP4
Note: It represent expression levels of BMP4 gene in whole embryos of E1–E6 and in brain, heart, liver and muscle of E9–E18 (Mean ± SD) (n=6), respectively. * or ** indicates significant difference between E1 and E2–E18 (P<0.05 or P<0.01).
图 3 BMP7基因的表达水平
注:图3表示BMP7基因在E1~E6整胚和E9~E18胚龄大脑、心脏、肝脏和腿肌中的表达水平(平均值±标准差)(n=6)。*或**表示BMP7基因在E2~E18胚龄中的表达水平与E1中的表达水平相比差异显著(P<0.05)或极显著(P<0.01)。
Figure 3. Expression of BMP7
Note: It represent expression levels of BMP7 gene in whole embryos of E1–E6 and in brain, heart, liver and muscle of E9–E18 (Mean ± SD) (n=6), respectively. * or ** indicates significant difference between E1 and E2–E18 (P<0.05 or P<0.01).
表 1 qPCR引物序列、扩增片段大小和退火温度
Table 1 Primer sequences, product sizes and annealing temperature applied for qPCR analysis
基因 Gene 引物序列(5′→3′)Primer sequence (5′→3′) 片段大小 Amplicon/bp 退火温度 Annealing temperature /℃ 登录号 Accession No. BMP2 TGGTGGAGGTGGTTCACTT 181 60 NM_204358 TTGTGTTTCGCTTGACGC BMP4 CTTCGTCTTCAACCTCAGC 150 59 NM_205237 ACAGCGGCTTCATCACTT BMP7 CTGATTTGTTCCTGCTCG 152 58 AF205877 GCTTTGCCCATCTATGCT GAPDH CTACACACGGACACTTCAAG 244 59 NM_204305 ACAAACATGGGGGCATCAG -
[1] 王广, 李艳, 杨雪松. 鸡胚早期发育过程中细胞迁移的基因调控 [J]. 中国细胞生物学学报, 2011, 33(10):1069−1077. WANG G, LI Y, YANG X S. Gene regulation of cell migration during the development of early chick embryo [J]. Chinese Journal of Cell Biology, 2011, 33(10): 1069−1077.(in Chinese)
[2] HOGAN B L. Bone morphogenetic proteins: multifunctional regulators of vertebrate development [J]. Genes & Development, 1996, 10(13): 1580−1594.
[3] CHEN D, ZHAO M, MUNDY G R. Bone morphogenetic proteins [J]. Growth Factors, 2004, 22(4): 233−241. DOI: 10.1080/08977190412331279890
[4] EVEN J, ESKANDER M, KANG J. Bone morphogenetic protein in spine surgery: current and future uses [J]. Journal of the American Academy of Orthopaedic Surgeons, 2012, 20(9): 547−52. DOI: 10.5435/JAAOS-20-09-547
[5] ISRAEL D I, NOVE J, KERNS K M, et al. Expression and characterization of bone morphogenetic protein-2 in Chinese hamster ovary cells [J]. Growth Factors, 1992, 7(2): 139−150. DOI: 10.3109/08977199209046403
[6] WOZNEY J M, ROSEN V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair [J]. Clinical Orthopaedics and Related Research, 1998, 346: 26−37.
[7] WOZNEY J M, ROSEN V, CELESTE A J, et al. Novel regulators of bone formation: molecular clones and activities [J]. Science, 1988, 242(4885): 1528−1534. DOI: 10.1126/science.3201241
[8] HE X N, DZIAK R, YUAN X, et al. BMP2 genetically engineered MSCs and EPCs promote vascularized bone regeneration in rat critical-sized calvarial bone defects [J]. PLoS One, 2013, 8(4): e60473. DOI: 10.1371/journal.pone.0060473
[9] LIU Z, YUAN X, FERNANDES G, et al. The combination of nano-calcium sulfate/platelet rich plasma gel scaffold with BMP2 gene-modified mesenchymal stem cells promotes bone regeneration in rat critical-sized calvarial defects [J]. Stem Cell Research & Therapy, 2017, 8(1): 122.
[10] SHIMASAKI S, ZACHOW R J, LI D, et al. A functional bone morphogenetic protein system in the ovary [J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(13): 7282−7287. DOI: 10.1073/pnas.96.13.7282
[11] SHIMASAKI S, MOORE R K, OTSUKA F, et al. The bone morphogenetic protein system in mammalian reproduction [J]. Endocrine Reviews, 2004, 25(1): 72−101. DOI: 10.1210/er.2003-0007
[12] ROBERT E GODIN, NORMA T TAKAESU, ELIZABETH J ROBERTSON, et al. Regulation of BMP7 expression during kidney development [J]. Development, 1998, 125(17): 3473−3482.
[13] TSENG Y H, KOKKOTOU E, SCHULZ T J, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure [J]. Nature, 2008, 454(7207): 1000−1004. DOI: 10.1038/nature07221
[14] SAINI S, DURAISAMY A J, BAYEN S, et al. Role of BMP7 in appetite regulation, adipogenesis, and energy expenditure [J]. Endocrine, 2015, 48(2): 405−409. DOI: 10.1007/s12020-014-0406-8
[15] SOMI S, BUFFING A A, MOORMAN A F, et al. Dynamic patterns of expression of BMP isoforms 2, 4, 5, 6, and 7 during chicken heart development [J]. Anatomical Record Part A-Discoveries in Molecular Cellular and Evolutionary Biology, 2004, 279(1): 636−651.
[16] SCHLUETER J, MÄNNER J, BRAND T. BMP is an important regulator of proepicardial identity in the chick embryo [J]. Developmental Biology, 2006, 295(2): 546−58. DOI: 10.1016/j.ydbio.2006.03.036
[17] GEETHA-LOGANATHAN P, NIMMAGADDA S, HUANG R, et al. Expression pattern of BMPs during chick limb development [J]. Anatomy and Embryology, 2006, 211(S1): 87−93. DOI: 10.1007/s00429-006-0129-6
[18] CHO T J, GERSTENFELD L C, EINHORN T A. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing [J]. Journal of Bone and Mineral Research, 2002, 17(3): 513−520. DOI: 10.1359/jbmr.2002.17.3.513
[19] KISHIGAMI S, MISHINA Y. BMP signaling and early embryonic patterning [J]. Cytokine & Growth Factor Reviews, 2005, 16(3): 265−278.
[20] ZHANG H, BRADLEY A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development [J]. Development, 1996, 122(10): 2977−2986.
[21] YU Y H, WILK K, WALDON P L, et al. In vivo identification of Bmp2-correlation networks during fracture healing by means of a limb-specific conditional inactivation of Bmp2 [J]. Bone, 2018, 116: 103−110. DOI: 10.1016/j.bone.2018.07.016
[22] SOUZA C J, CAMPBELL B K, MCNEILLY A S, et al. Effect of bone morphogenetic protein 2(BMP2) on oestradiol and inhibin A production by sheep granulosa cells, and localization of BMP receptors in the ovary by immunohistochemistry [J]. Reproduction, 2002: 363−369. DOI: 10.1530/rep.0.1230363
[23] JUENGEL J L, READER K L, BIBBY A H, et al. The role of bone morphogenetic proteins 2, 4, 6 and 7 during ovarian follicular development in sheep: contrast to rat [J]. Reproduction, 2006, 131: 501−513. DOI: 10.1530/rep.1.00958
[24] ONAGBESAN O M, BRUGGEMAN V, VAN A P, et al. BMPs and BMPRs in chicken ovary and effects of BMP-4 and -7 on granulosa cell proliferation and progesterone production in vitro [J]. American Journal of Physiology and Endocrinological Metabolism, 2003, 285(5): E973−E983. DOI: 10.1152/ajpendo.00104.2003
[25] ZHANG Z, DI R, LIU Q, et al. Expression analysis of five genes in the gonadal axis of Small Tail Han sheep and Sunite sheep [J]. Scientia Agricultura Sinica, 2018, 51: 4710−4719.
[26] MOWBRAY C, HAMMERSCHMIDT M, WHITFIELD T T. Expression of BMP signalling pathway members in the developing zebrafish inner ear and lateral line [J]. Mechanisms of Development, 2001, 108(1/2): 179−184.
[27] LA ROSA I, CAMARGO L S, PEREIRA M M, et al. Effects of bone morphogenic protein 4(BMP4) and its inhibitor, Noggin, on in vitro maturation and culture of bovine preimplantation embryos [J]. Reproductive Biology and Endocrinology, 2011, 9(1): 18. DOI: 10.1186/1477-7827-9-18
[28] KIM D, OCÓN-GROVE O, JOHNSON A L. Bone morphogenetic protein 4 supports the initial differentiation of hen (Gallus gallus) granulosa cells [J]. Biology of Reproduction, 2013, 88(6): 161. DOI: 10.1095/biolreprod.113.109694
[29] SU S Y, DONG Z J. Comparative expression analyses of bone morphogenetic protein 4(BMP4) expressions in muscles of Tilapia and common carp indicate that BMP4 plays a role in the intermuscular bone distribution in a dose-dependent manner [J]. Gene Expression Patterns, 2018, 27: 106−113. DOI: 10.1016/j.gep.2017.11.005
[30] YUAN J S, DENG Y, ZHANG Y Y, et al. Bmp4 inhibits goose granulosa cell apoptosis via PI3K/AKT/Caspase-9 signaling pathway [J]. Animal Reproduction Science, 2019, 200: 86−95. DOI: 10.1016/j.anireprosci.2018.11.014
[31] BRAGDON B, MOSEYCHUK O, SALDANHA S, et al. Bone Morphogenetic Proteins: A critical review [J]. Cellular Signalling, 2011, 23(4): 609−620. DOI: 10.1016/j.cellsig.2010.10.003
[32] KUSAKAWA Y, MIKAWA S, SATO K. BMP7 expression in the adult rat brain [J]. IBRO Reports, 2017, 3: 72−86. DOI: 10.1016/j.ibror.2017.06.002
[33] CAMBRIA M T, VILLAGGIO G, FEDERICO C, et al. Bone morphogenic protein BMP7 induces adipocyte differentiation and uncoupling protein UCP1 expression in human bone marrow mesenchymal stem cells [J]. Rendiconti Lincei, 2017, 28(4): 635−641. DOI: 10.1007/s12210-017-0643-x
[34] DONG X G, LI J Y, ZHANG Y Y, et al. Genomic analysis reveals pleiotropic alleles at EDN3 and BMP7 involved in chicken comb color and egg production [J]. Frontiers in Genetics, 2019, 10: 612. DOI: 10.3389/fgene.2019.00612
[35] JIN W Z, TAKAGI T, KANESASHI S N, et al. Schnurri-2 controls BMP-dependent adipogenesis via interaction with smad proteins [J]. Developmental Cell, 2006, 10(4): 461−471. DOI: 10.1016/j.devcel.2006.02.016
[36] RAJESH G, MISHRA S R, PAUL A, et al. Transcriptional and translational abundance of bone morphogenetic protein (BMP) 2, 4, 6, 7 and their receptors BMPR1A, 1B and BMPR2 in buffalo ovarian follicle and the role of BMP4 and BMP7 on estrogen production and survival of cultured granulosa cells [J]. Research in Veterinary Science, 2018, 118: 371−388. DOI: 10.1016/j.rvsc.2018.04.002
[37] ZHANG Y L, LI F Z, FENG X, et al. Genome-wide analysis of DNA Methylation profiles on sheep ovaries associated with prolificacy using whole-genome Bisulfite sequencing [J]. BMC Genomics, 2017, 18: 759. DOI: 10.1186/s12864-017-4068-9
[38] ZHANG Z B, LIU Q Y, DI R, et al. Single nucleotide polymorphisms in BMP2 and BMP7 and the association with litter size in Small Tail Han sheep [J]. Animal Reproduction Science, 2019, 204: 183−192. DOI: 10.1016/j.anireprosci.2019.04.001
-
期刊类型引用(0)
其他类型引用(1)




 下载:
下载: