Establishment and application of multiplex RT-PCR methods for akabane virus, bovine herpesvirus 1 and bovine viral diarrhea virus
-
摘要:
目的 旨在建立一种可同时检测赤羽病病毒(Akabane virus, AKAV)、牛疱疹病毒1型(Bovine herpesvirus 1, BoHV-1)和牛病毒性腹泻病毒(Bovine viral diarrhea virus, BVDV)的多重RT-PCR检测方法,为牛繁殖障碍类疫病鉴别诊断及防控奠定基础。 方法 针对AKAV S基因、BoHV-1 gH基因和BVDV 3'UTR基因保守区域设计3对引物,通过反应条件参数的优化,建立AKAV、BoHV-1和BVDV多重RT-PCR检测方法。 结果 特异性结果显示,该方法仅对AKAV、BoHV-1和BVDV阳性样品检测为阳性,对牛细小病毒(BPV)、牛呼吸道合胞体病毒(BRSV)、牛副流感病毒3型(BPIV3)、口蹄疫病毒(FMDV)和牛流行热病毒(BEFV)等阳性样品检测为阴性。敏感性结果显示,该方法对AKAV、BoHV-1和BVDV重组质粒的下限阈值均为1.0×103 拷贝·μL−1。重复性结果显示,批内、批间重复性均较好。利用建立的方法对143份患繁殖障碍的牛全血/组织样品进行测定,并与现有的地方标准进行对比,验证本检测方法的实际临床应用效能。结果显示,AKAV、BoHV-1和BVDV的阳性率分别为2.80%(4/143)、21.68%(31/143)、38.46%(55/143),存在混合感染现象,与AKAV、BoHV-1和BVDV地方标准的符合率为99.3%、98.6%和97.2%,Kappa系数分别为0.885、0.960和0.942,表明本研究建立的方法与地方标准两者一致性良好。 结论 本研究开创性地建立了一种特异性强、灵敏度高和成本低的多重RT-PCR检测方法,适用于AKAV、BoHV-1和BVDV的鉴别检测。 Abstract:Objective To establish a multiplex RT-PCR method for the rapid and simultaneous detection of akabane virus (AKAV), Bovine herpesvirus 1 (BoHV-1) and bovine viral diarrhea virus (BVDV), so as to lay a foundation for the differential diagnosis, prevention and control of bovine reproductive disorder epidemics. Method Three pairs of primers were designed for the conservative regions of AKAV S gene, BoHV-1 gH gene and BVDV 3'UTR gene, and a multiplex RT-PCR assay for AKAV, BoHV-1 and BVDV was established through the optimal of reaction condition parameters. Result The specificity results showed that the method was positive only for AKAV, BoHV-1 and BVDV, and negative for the nucleic acids of bovine parvovirus (BPV), bovine respiratory syncytial virus (BRSV), bovine parainfluenza virus 3 (BPIV3), foot-and-mouth disease virus (FMDV), and bovine ephemeral fever virus (BEFV). The sensitivity results showed that the lower threshold of this method for AKAV, BoHV-1 and BVDV recombinant plasmids were all 1.0×103 copies/μL. The results of the reproducibility test showed good intra- and inter-batch reproducibility. The established method was used to determine 143 whole blood/tissue samples of cattle with reproductive disorders and compared with the existing local standards to verify the efficacy of this test for practical clinical application. The results showed that the positivity rates of AKAV, BoHV-1 and BVDV were 2.80% (4/143), 21.68% (31/143) and 38.46% (55/143), respectively, with the presence of several mixed infections; and the conformity rates of this method with the local standards for AKAV, BoHV-1 and BVDV were 99.3%, 98.6% and 97.2%, respectively. The Kappa coefficients of the two were 0.885, 0.960 and 0.942 respectively, indicating that the method established in this study was in good agreement with the local standards. Conclusion In this study, we pioneered a multiplex RT-PCR assay with high specificity, sensitivity and low cost for the differential detection of AKAV, BoHV-1 and BVDV. -
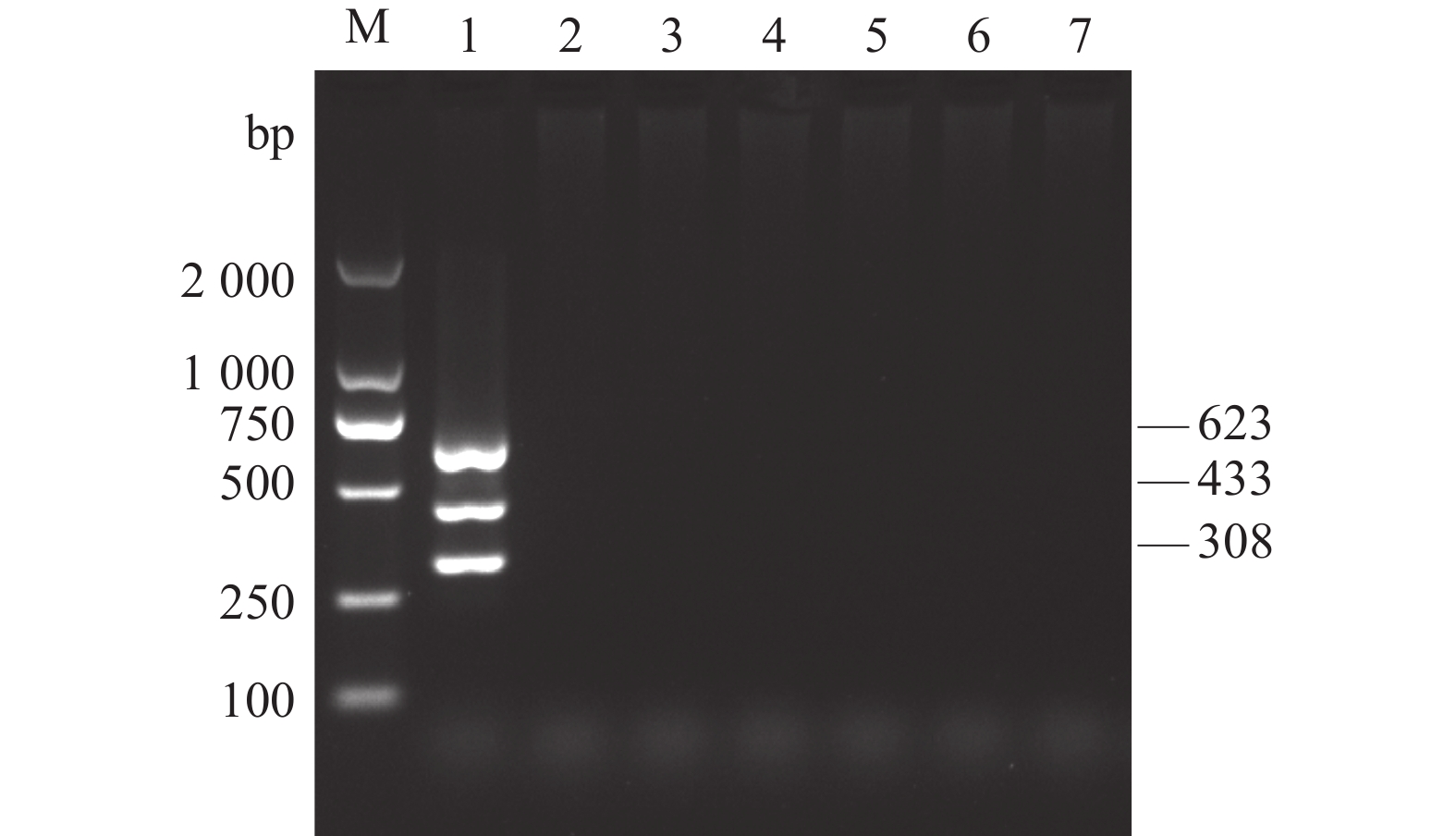
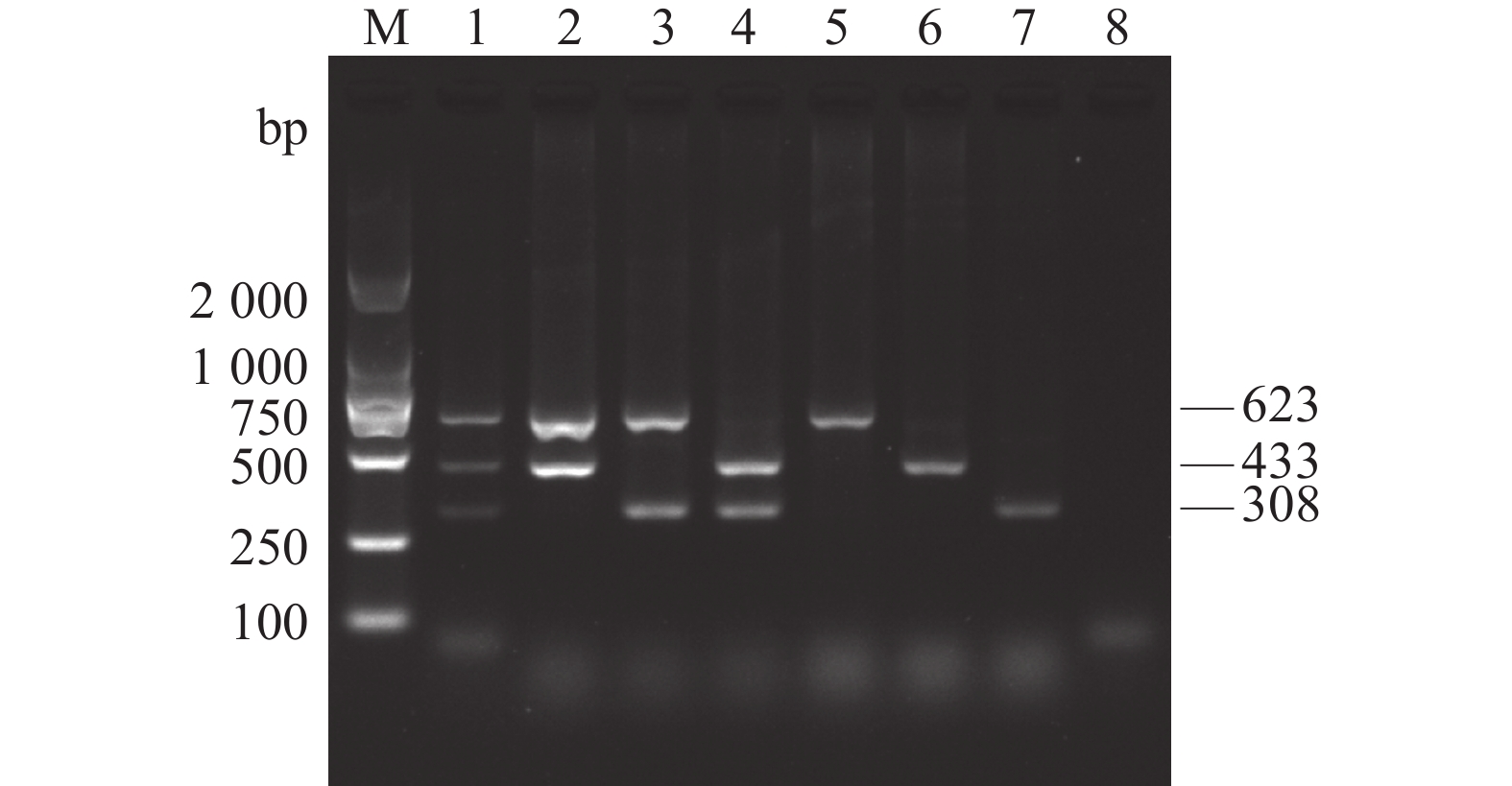
图 1 AKAV、BoHV-1和BVDV单重和多重PCR扩增
M:DL2 000 DNA Marker;1:AKAV、BoHV-1和BVDV;2:AKAV和BoHV-1;3:AKAV和BVDV;4:BoHV-1和BVDV;5:AKAV;6:BoHV-1;7:BVDV;8:阴性对照。
Figure 1. Single and multiplex PCR products of AKAV, BoHV-1 and BVDV
1: AKAV, BoHV-1 and BVDV; 2: AKAV and BoHV-1; 3: AKAV and BVDV; 4: BoHV-1 and BVDV; 5: AKAV; Lane 6: BoHV-1; Lane 7: BVDV; 8: negative control; M: DL2 000 DNA Marker.
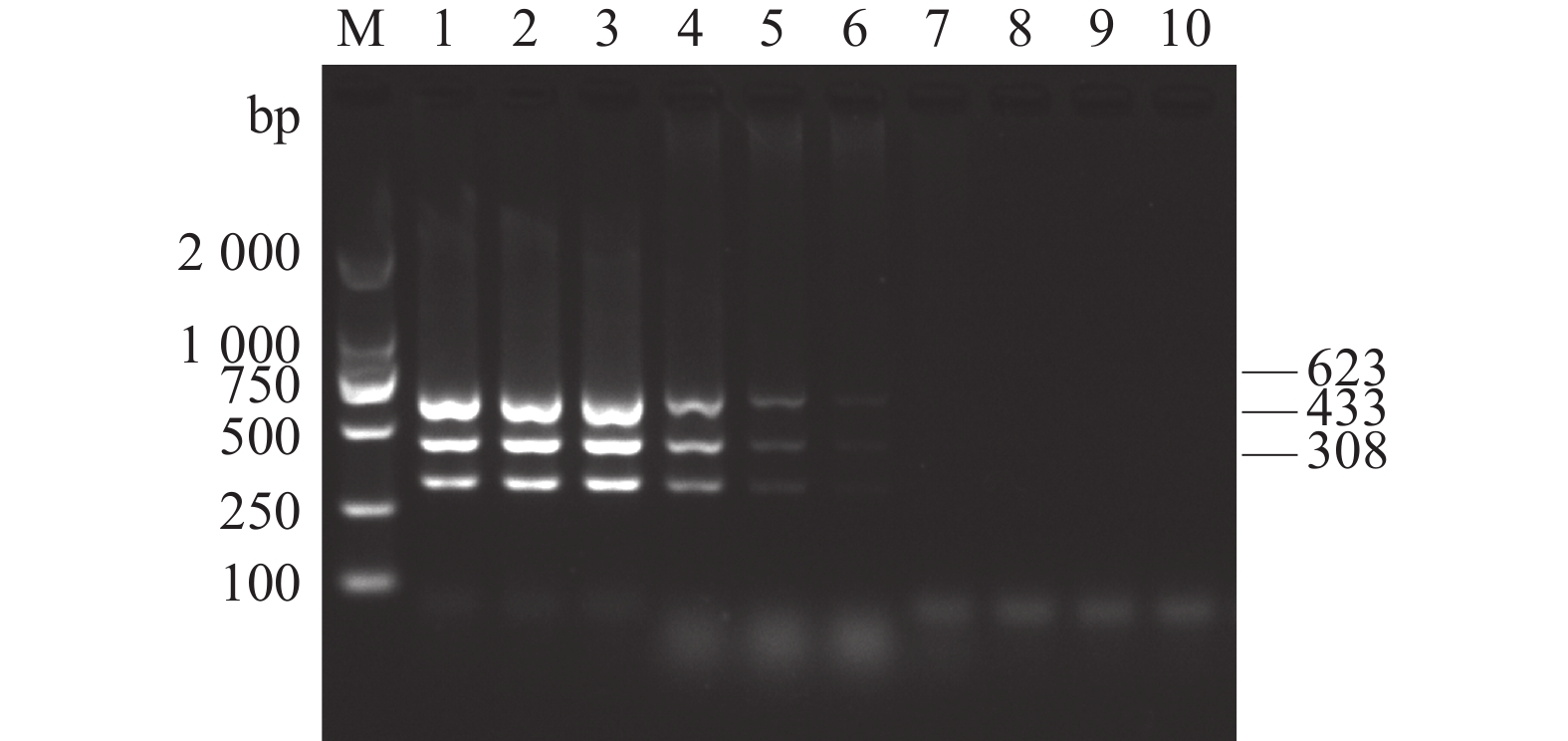
图 2 AKAV、BoHV-1和BVDV多重PCR特异性试验
M:DL2 000 DNA Marker;1:AKAV、BoHV-1和BVDV多重PCR扩增产物; 2~6分别为BPV、BRSV、BPIV3、FMDV和BEFV;7:阴性对照。
Figure 2. Validation of the specificity of the multiplex PCR for BoHV-1, AKAV and BVDV detection
M: DL2 000 DNA Marker; 1: BoHV-1, AKAV and BVDV; 2–6: BPV, BRSV, BPIV3, FMDV and BEFV, respectively; 7: negative control.
表 1 引物序列Fig.1 The information of primer sequences
病毒
Virus引物序列(5'-3')
Sequences of the primers for PCR(5'-3')靶基因
Target gene产物大小(bp)
Products size/bpAKAV AKAV-F:ACATAAGACGCCACAACCAAGT S 623 AKAV-R:CGAGCAGCTGAACAAAGGTG BoHV-1 BoHV-1-F:TCCTGCCTCTGGAGTTTATGG gH 433 BoHV-1-R:TTGAGGTGGTAGTCAAGGTCG BVDV BVDV-F:CAGCGAAGGCCGAAAAGAGG 3'UTR 308 BVDV-R:CCATGTGCCATGTACAGCAGAG 表 2 临床样本检测结果对比
Table 2. Comparison results of positive rates of two methods
病毒
Virus本研究方法
This study地方标准检测方法
Local standardsKappa值
Kappa value阳性样本数
No. of positive samples阳性率
Positivity rate阳性样本数
No. of positive samples阳性率
Positivity rate赤羽病病毒 AKAV 4 2.80% 5 3.50% 0.8852 牛疱疹病毒1型 BoHV-1 31 21.68% 33 23.08% 0.9597 牛病毒性腹泻病毒 BVDV 55 38.46% 59 41.26% 0.9417 -
[1] ASMARE K, SIBHAT B, AYELET G, et al. Serological evidence of Bovine herpesvirus-1, Bovine Viral Diarrhea virus and Schmallenberg virus infections in relation to reproductive disorders in dairy cattle in Ethiopia [J]. Acta Tropica, 2018, 178: 236−241. [2] OGAWA Y, FUKUTOMI T, SUGIURA K, et al. Comparison of Akabane virus isolated from sentinel cattle in Japan [J]. Veterinary Microbiology, 2007, 124(1/2): 16−24. [3] 朱杰, 杨才俊, 祁明普, 等. 我国牛群中牛病毒性腹泻病毒流行的Meta分析 [J]. 华中农业大学学报, 2023, 42(2):48−62.ZHU J, YANG C J, QI M P, et al. Meta analysis of prevalence of bovine viral diarrhea virus(BVDV)in cattle in China [J]. Journal of Huazhong Agricultural University, 2023, 42(2): 48−62. (in Chinese) [4] NANDI S, KUMAR M, MANOHAR M, et al. Bovine herpes virus infections in cattle [J]. Animal Health Research Reviews, 2009, 10(1): 85−98. doi: 10.1017/S1466252309990028 [5] MAHMOUD H, ALI A. Epidemiological investigation on Bovine alphaherpesvirus 1 and bovine viral diarrhea virus in cattle and camels in southern Egypt[J]. Veterinaria Italiana, 2022, 58(4). doi: 10.12834/VetIt.2361.14459.2. [6] KUMAR N, CHANDER Y, RIYESH T, et al. Isolation and characterization of bovine herpes virus 5 (BoHV5) from cattle in India [J]. PLoS One, 2020, 15(4): e0232093. doi: 10.1371/journal.pone.0232093 [7] YANG G H, ZOU Y J, YANG R J, et al. A bovine viral diarrhea virus type 1c strain in China: Isolation, identification, and assessment of pathogenicity in rabbits [J]. Current Microbiology, 2022, 79(12): 356. doi: 10.1007/s00284-022-03069-z [8] WANG J, YIN J H, WANG S H, et al. Development and application of an indirect ELISA for the serological detection of bovine viral diarrhea virus infection based on the protein E2 antigen [J]. Molecular Biology Reports, 2023, 50(5): 4707−4713. doi: 10.1007/s11033-022-08226-y [9] BERTOLOTTI L, MURATORE E, NOGAROL C, et al. Development and validation of an indirect ELISA as a confirmatory test for surveillance of infectious bovine rhinotracheitis in vaccinated herds [J]. BMC Veterinary Research, 2015, 11: 300. doi: 10.1186/s12917-015-0612-5 [10] DONG S J, FENG M, YU R S, et al. Establishment and application of visual LAMP detection method of infectious bovine rhinotracheitis virus [J]. Chinese Journal of Biotechnology, 2018, 34(10): 1587−1595. [11] KAMATA H, INAI K, MAEDA K, et al. Encephalomyelitis of cattle caused by akabane virus in southern Japan in 2006 [J]. Journal of Comparative Pathology, 2009, 140(2/3): 187−193. [12] BROCK J, LANGE M, GUELBENZU-GONZALO M, et al. Epidemiology of age-dependent prevalence of Bovine Herpes Virus Type 1 (BoHV-1) in dairy herds with and without vaccination [J]. Veterinary Research, 2020, 51(1): 124. doi: 10.1186/s13567-020-00842-5 [13] 卞志标, 郭怡德, 席振军, 等. 非洲猪瘟病毒防污染双重荧光定量PCR检测方法的建立 [J]. 动物医学进展, 2023, 44(1):19−26. doi: 10.3969/j.issn.1007-5038.2023.01.004BIAN Z B, GUO Y D, XI Z J, et al. Establishment of ASFV double fluorescent quantitative PCR assay for pollution prevention [J]. Progress in Veterinary Medicine, 2023, 44(1): 19−26. (in Chinese) doi: 10.3969/j.issn.1007-5038.2023.01.004 [14] 白泉阳, 徐磊, 傅光华, 等. 检测猪源牛病毒性腹泻病毒荧光RT-PCR方法的建立与监测分析 [J]. 福建农业学报, 2017, 32(8):828−832.BAI Q Y, XU L, FU G H, et al. Establishment and monitoring analysis of fluorescence RT-PCR for detection of bovine viral diarrhea virus in swine [J]. Fujian Journal of Agricultural Sciences, 2017, 32(8): 828−832. (in Chinese) [15] 孙映雪, 宋建德, 郑雪光, 等. 2020—2022年全球牛结节性皮肤病流行状况 [J]. 中国动物检疫, 2023, 40(5):1−4. doi: 10.3969/j.issn.1005-944X.2023.05.001SUN Y X, SONG J D, ZHENG X G, et al. Global prevalence of lumpy skin disease from 2020 to 2022 [J]. China Animal Health Inspection, 2023, 40(5): 1−4. (in Chinese) doi: 10.3969/j.issn.1005-944X.2023.05.001 [16] HOU P L, ZHAO G M, WANG H M, et al. Prevalence of bovine viral diarrhea virus in dairy cattle herds in Eastern China [J]. Tropical Animal Health and Production, 2019, 51(4): 791−798. doi: 10.1007/s11250-018-1751-z [17] ZHANG F F, LI H Q, LIN C, et al. Detection and genetic diversity of subgroup K avian leukosis virus in local chicken breeds in Jiangxi from 2021 to 2023 [J]. Frontiers in Microbiology, 2024, 15: 1341201. doi: 10.3389/fmicb.2024.1341201 [18] ZHANG F F, XIE Q, YANG Q, et al. Prevalence and phylogenetic analysis of Gyrovirus galga 1 in Southern China from 2020 to 2022 [J]. Poultry Science, 2024, 103(3): 103397. [19] HOU P L, XU Y R, WANG H M, et al. Detection of bovine viral diarrhea virus genotype 1 in aerosol by a real time RT-PCR assay [J]. BMC Veterinary Research, 2020, 16(1): 114. doi: 10.1186/s12917-020-02330-6 [20] HWANG S, LEE W, LEE Y. Development of a nucleic acid detection method based on the CRISPR-Cas13 for point-of-care testing of bovine viral diarrhea virus-1b [J]. Journal of Animal Science and Technology, 2024, 66(4): 781−791. doi: 10.5187/jast.2023.e77 [21] 鲍显伟, 李小龙, 石亚楠, 等. 牛传染性鼻气管炎病毒和牛病毒性腹泻病毒双重PCR检测方法的建立与应用 [J]. 中国动物检疫, 2023, 40(1):115−120. doi: 10.3969/j.issn.1005-944X.2023.01.021BAO X W, LI X L, SHI Y N, et al. Establishment and application of a dual PCR assay for detection of infectious bovine rhinotracheitis virus and bovine viral diarrhea virus [J]. China Animal Health Inspection, 2023, 40(1): 115−120. (in Chinese) doi: 10.3969/j.issn.1005-944X.2023.01.021 [22] MENG W X, CHEN Z H, JIANG Q F, et al. A multiplex real-time fluorescence-based quantitative PCR assay for calf diarrhea viruses [J]. Frontiers in Microbiology, 2024, 14: 1327291. doi: 10.3389/fmicb.2023.1327291 -







 下载:
下载: